Important 2024 Blue Advantage® Formulary Updates
Blue Advantage® has some important 2024 formulary updates for you to consider for your patients.
Insulin Coverage Changes for 2024
Effective January 1, 2024, certain long-acting insulins covered on the Blue Advantage formulary will be updated as follows:

Note: Novo Nordisk will discontinue its long-acting insulin Levemir in the U.S. in 2024.
- Supply disruptions of Levemir FlexPen are expected in mid-January 2024.
- Levemir FlexPen will be discontinued on April 1, 2024.
- Levemir vial will be discontinued on December 31, 2024.
Lantus and Toujeo are alternative covered long-acting insulins for the 2024 formulary.
Reference: https://www.mynovoinsulin.com/insulin-products/levemir/home.html
If switching patients to a covered insulin (Lantus and Toujeo), use the chart below to help with this transition. These alternative options are on the 2023 Blue Advantage formularies so you can begin switching affected patients before 2024.
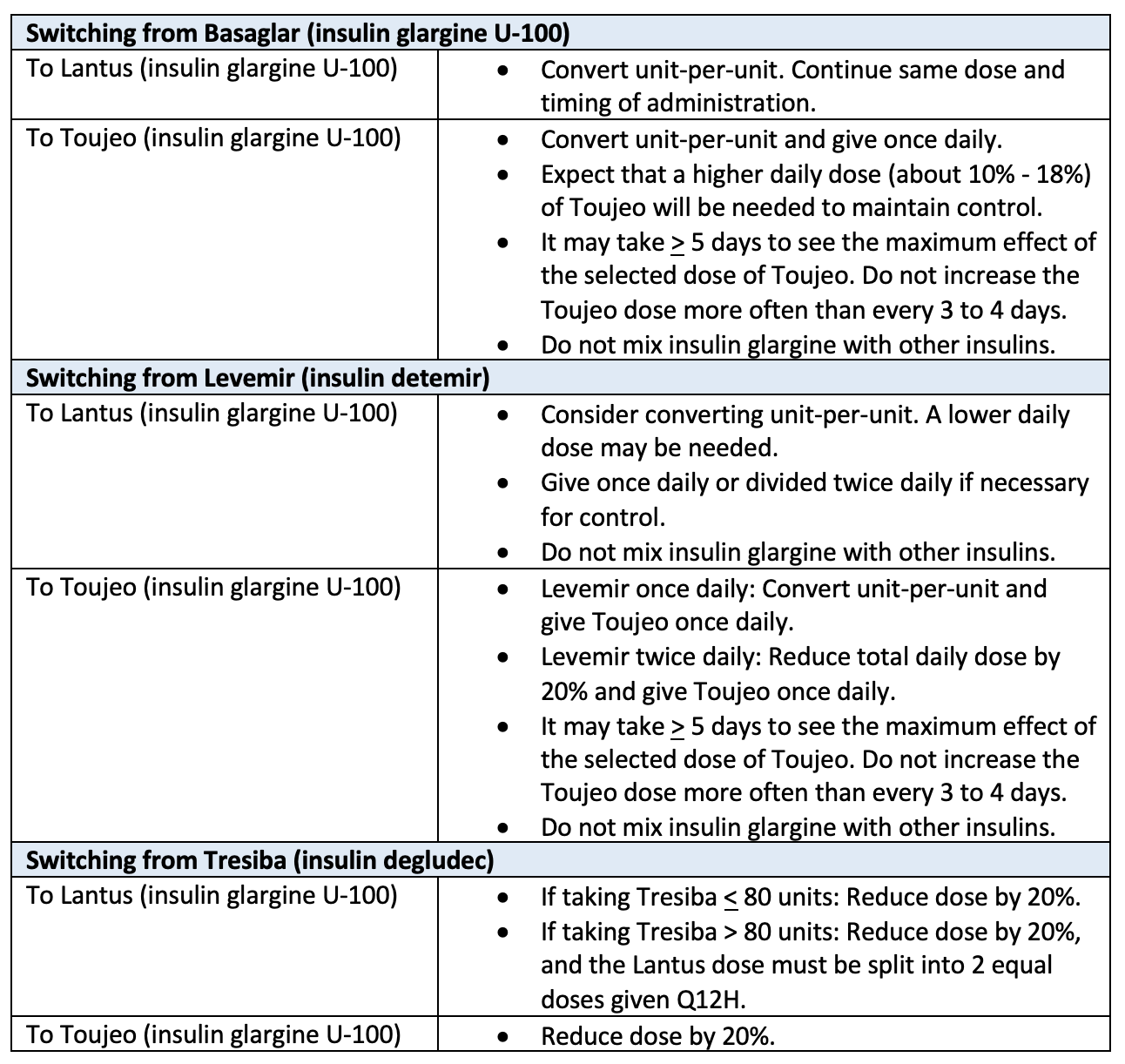
Alternatively, you can submit a formulary exception for each of your impacted patients to ensure their insulin regimen is not interrupted. Formulary exceptions for 2024 can be submitted starting November 1, 2023. Formulary exceptions can be submitted through Prime Therapeutics or CoverMyMeds.
Formulary exceptions require that a member must have tried and failed two formulary products or have clinical justification for not being able to use any of the formulary products in order to be approved. Formulary exceptions must be designated as a 2024 request.
Pulmonary Agents Coverage Change for 2024
Effective January 1, 2024, Blue Advantage will no longer include brand-name pulmonary agents Advair Diskus and Flovent HFA. Instead, their generic alternatives will be covered: fluticasone/salmeterol and fluticasone propionate hfa.
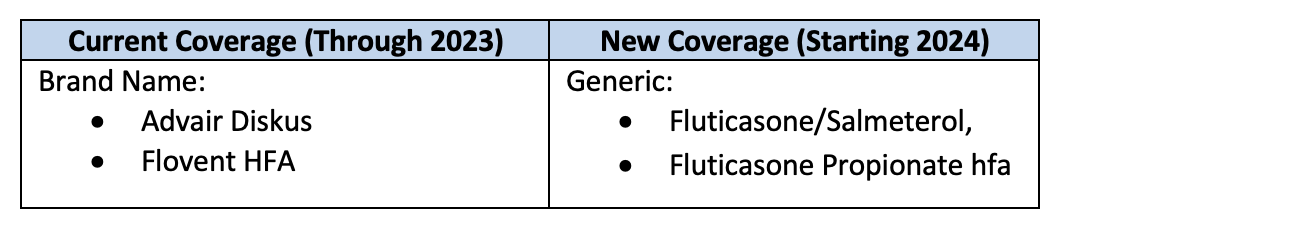
Patients with a prescription for one of these inhalers will automatically be switched to the generic version at the point of sale, unless the original prescription indicates “dispense as written.” If the original prescription indicates “dispense as written,” a new prescription will be required to switch to the generic alternative.
If a patient requires the brand name, providers can begin submitting a formulary exception request for 2024 starting on November 1, 2023. Formulary exceptions can be submitted through Prime Therapeutics or CoverMyMeds. Additionally, Symbicort, and its generic (budesonide/formoterol), will not be covered on the Blue Advantage formularies in 2024.
Formulary exceptions require that a member must have tried and failed two formulary products or have clinical justification for not being able to use any of the formulary products in order to be approved. Formulary exceptions must be designated as a 2024 request.
CoverMyMeds is available to Patrius Health through Prime Therapeutics, an independent company providing pharmacy benefit management services. CoverMyMeds is an independent company that provides electronic solutions for drug prior authorizations.
